C. Cristiani, M. Bellotto, G. Dotelli, S. Latorrata, G. Ramis, P. Gallo Stampino, E.M. Iannicelli-Zubiani, E. Finocchio
Minerals, 11(1) (2021), 1-15
https://doi.org/10.3390/min11010030
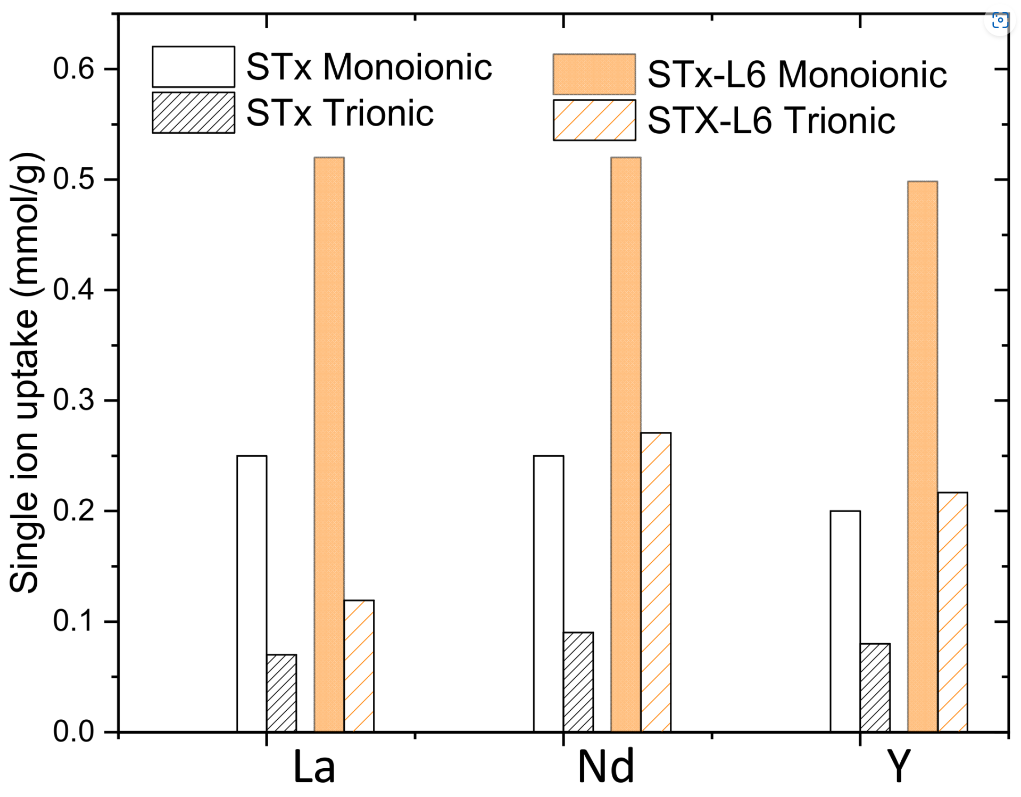
Metals from electric and electronic waste equipment (WEEE) can be recovered by dissolution with acids followed by liquid–liquid extraction. A possible alternative to liquid–liquid extraction is liquid–solid adsorption, where sorbents efficiency is the key factor for process efficiency. In this respect, aim of this paper is the study of the behaviour of two solid sorbents for the recovery of Rare Earths (REs)—in particular, La, Nd, and Y—from scraps of end-of-Life (EOL) electronic equipment. Two solid matrices were considered: a pristine montmorillonite clay and a modified-montmorillonite clay intercalated with a commercial pentaethylen-hexamine. The capture ability of the solids was tested towards single-ion La, Nd, and Y solutions and a multi-element solution containing the three ions. Before and after the uptake step, samples of both the solid and liquid phases were analysed. For both sorbents, at lower metal initial concentrations, the ions were captured in similar amount. At higher concentrations, pure clay showed a high total uptake towards La ions, likely due to surface interactions with clay sites. The organoclay preferentially interacts with Nd and Y. Considering the presence of the polyamine, this behaviour was related to ion coordination with the amino groups. The capture behaviour of the two sorbents was related to the different physicochemical properties of the ions, as well as to the ionic radius.