C. Cristiani, M. Bellotto, G. Dotelli, P. Gallo Stampino, S. Latorrata, E. Finocchio
Polymers, 14(3) (2022), 485
https://doi.org/10.3390/polym14030485
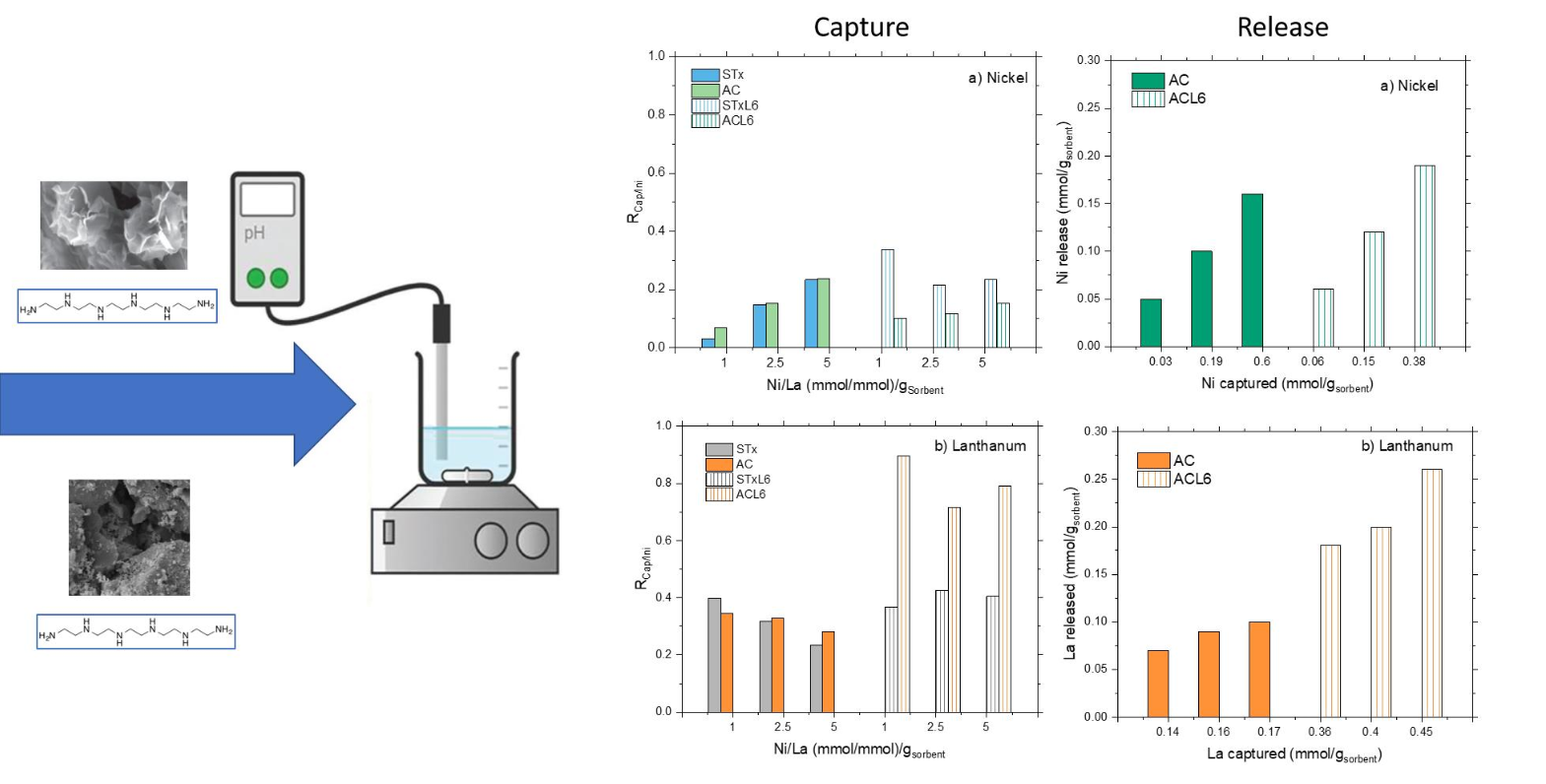
This study investigated on a laboratory scale the capability of mineral clays (STx) and activated carbons (AC), both pristine and modified (STx-L6 and AC-L6) with a linear penta-ethylene-hexamine (L6), to capture and release rare earths (REs) and transition metals (TMs) from solutions representative of a hydrometallurgical process. The aim is to develop an efficient method for the recovery of such maeterials from waste electrical and electronic equipment (WEEE). A liquid/solid adsorption process has been exploited, by contacting the solids with synthetic mono- and bi-ionic solutions containing Ni(II) and La(III). The experiments were conducted at room temperature for 90 min by keeping a fixed La concentration of 19 mM and varying the one of Ni in the 19–100 mM range. Ni(II) and La(III) were captured by the four solids, both in single- and bi-ionic solutions; nevertheless, the presence of the polyamine always improved significantly the capture ability of the pristine sorbents. For all the four solids, the capture behaviour can be ascribed to an adsorption or ion-sorbent interaction process, since no aquo- and hydroxy-Ni or La can be formed. The polyamine, which is able to capture Ni ions via coordination, allowed to differentiate the behaviour of ion capture, bypassing the direct competition between Ni and La ions for the capture sites found in the pristine solids. Release values of 30 up to 100% were found following a one-step treatment with a concentrated HNO3 solution. However, the recovery of different metals was found also in this case, depending on both sorbent and ions, which suggests a possible selective recovery.